The start of the love affair between mankind and gold is believed to be around –3000-6000 BC with the smelting of gold. According to Studies in Ancient Technology by R.J. Forbes, the technique began in Mesopotamia or Syria. In ancient Greece, Heraclitus wrote on the subject.
According to the Encyclopedia Britannica, ‘Egyptian wall reliefs from 2,300 BC show gold in various stages of refining and mechanical working’, using ancient technology. Ancient Egyptians extracted gold using placer mining at the banks of rivers — a process in which valuable minerals that have accumulated through their respective density during sedimentary processes are mined from the banks of river beds. In other words, Egypt made use of particles of gold that they found in river sands.

Once the sand is collected, the gold is concentrated by washing away lighter river sands with water, thus leaving behind dense gold particles. These particles were further purified through melting and consequently moulded into the desired shapes. Forbes documented that by 2000 BC, the process of purifying alloys with salt to remove the silver and leave behind gold was discovered.
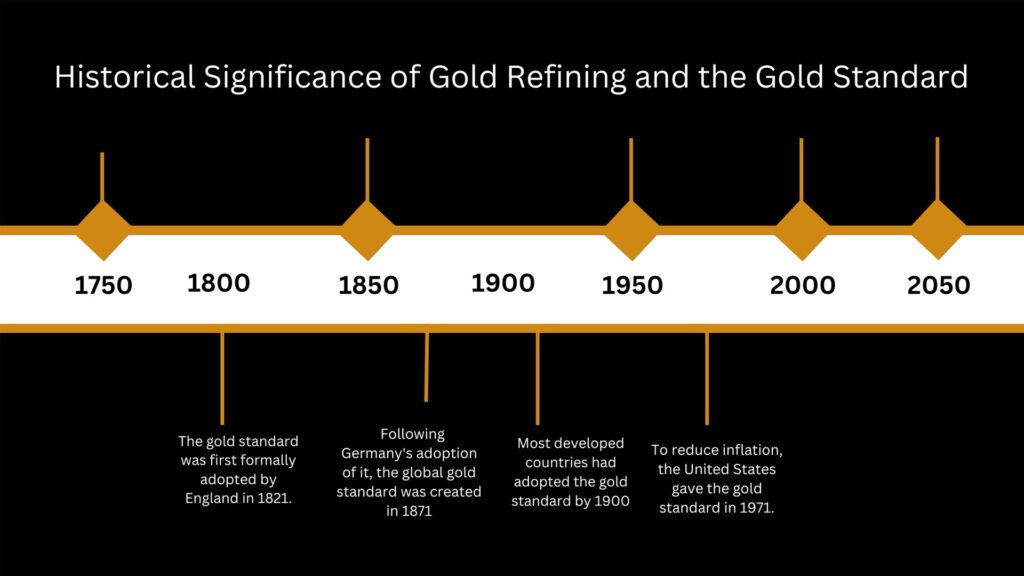
These techniques of refining gold eventually reached the Spanish by 100 AD, who may have been just as mystified with the precious metal as Indians are today. Gold Ore Processing: Project Development and Operations, edited by Mike D. Adams speculated that up to 40,000 slaves were employed for gold mining in Spain at the time.
The advent of Christianity somewhat tempered the demand for gold until about the 10th century AD, which is when amalgamation was discovered – the process using mercury to capture tiny particles of gold from sand. And soon after that, gold mining saw an exponential rise with the invasion of the New World. Soon, large scale gold mining using newer technologies spread to areas where gold deposits were discovered in Brazil, the Ural Mountains of Russia, Siberia and, California and the largest of which was in the Witwatersrand of South Africa.

The discovery of the South African gold deposit coincided with the discovery of the cyanidation process, which made it possible to recover gold from low-grade ores, and is the most commonly used leaching process for gold extraction today.
The technology boom for developing pure gold continued with E.B. Miller’s process of refining impure gold with chlorine gas (patented in Britain in 1867) and Emil Wohlwill’s electro-refining process (introduced in Hamburg, Germany, in 1878). In fact, gold with purity of 99.99 percent is created using a combination of the processes.
Suddenly, it became easier and cheaper to routinely purify gold. These two methods continue to be the most popular gold refining methods used today.
Gold refining has evolved over millennia, moving from simple separation methods to sophisticated industrial and chemical processes, driven by the need to increase purity for coinage, jewelry, and investment.

Ancient to Medieval Period (c. 3000 BCE – 1500s CE)
The primary method for purifying gold in the ancient world was cupellation, known as early as 2500 BCE in Mesopotamia and widely used by the Romans and throughout the Middle Ages.
- Cupellation: This process removes impurities (like lead, silver, copper, and tin) by placing the impure gold in a porous, cup-shaped vessel called a cupel (made of bone ash or clay) and heating it to high temperatures while blowing air over it. The impurities oxidize and are absorbed into the cupel or vaporize, leaving behind a bead of relatively pure gold.
- Parting (Quartation): To separate silver from gold (which cannot be done effectively through cupellation), the method of parting was used. The alloy was first melted with a large amount of silver (to ensure a 3:1 ratio of silver to gold) and then treated with strong acids or chemicals. In ancient times, this involved using a cementation process with salt and brick dust, which converted the silver into soluble silver chloride, leaving the pure gold behind. This method was widely used during the Middle Ages.
Post-Middle Ages and the Industrial Revolution (c. 1500s – 1800s)
While cupellation remained a staple, the focus shifted towards more efficient chemical parting methods as chemical knowledge advanced.
- Sulphuric Acid Parting: Introduced around the 1820s, this method was more efficient than the cementation process. Impure gold was boiled in concentrated sulphuric acid, which dissolved the silver and other base metals, leaving the gold unaffected.
Modern Period (1800s – Present)
The late 19th century introduced modern, large-scale industrial refining processes that are still in use today.
- Miller Process (1860s): Developed by Francis Bowyer Miller, this is the first modern high-speed refining method. Chlorine gas is bubbled through molten gold. Base metals and silver react with the chlorine to form chlorides, which rise to the surface as slag or vaporize. The resulting gold is approximately 99.5% pure, and the process is relatively fast.
- Wohlwill Process (1874): Developed by Emil Wohlwill, this is an electrolytic process used when extremely high purity gold is required (up to 99.999%). The impure gold acts as the anode, and a pure gold cathode is used. When an electric current is passed through a hydrochloric acid-based electrolyte, gold ions migrate from the anode and deposit as highly pure gold on the cathode.
- Aqua Regia Refining: This highly effective method involves dissolving the gold in aqua regia (a mixture of concentrated nitric acid and hydrochloric acid) and then precipitating the pure gold out of the solution using a chemical agent like ferrous sulfate or sulfur dioxide.
Today, large-scale refineries primarily use a combination of the Miller and Wohlwill processes to produce investment-grade gold bars (Good Delivery standards).

The history of gold refining showcases a progression from simple fire-based separation to modern, precise chemical and electrochemical methods. The fundamental goal has always been the removal of impurities, primarily base metals and silver, to increase purity.
Ancient and Pre-Modern Methods (c. 3000 BCE – 1800s CE)
Early gold refining methods were primarily physical and fire-based, as gold was often found in native, relatively pure forms.
- Fire Smelting: This is one of the oldest methods, dating back to at least 3000 BCE in ancient Egypt and Mesopotamia. Raw gold was heated in a crucible to high temperatures, allowing some impurities (dross) to be skimmed off the top or absorbed by added fluxing agents like borax or soda ash.
- Cupellation: This ancient process, also used by the Romans and in other early civilizations, effectively separates gold and silver from base metals.
- The impure gold (or gold-lead alloy) was heated in a specialized, porous cupel (often made of bone ash) under an oxidizing atmosphere.
- Base metals would oxidize and be absorbed by the porous cupel or vaporize, leaving behind a bead of a purer gold-silver alloy.
- Cementation: This method was used to part gold and silver. The gold-silver alloy was hammered into thin sheets, layered with a mixture of salt, brick dust, and sometimes other sulfates, and then heated. The salt would react with the silver to form silver chloride, which could be washed away, leaving the refined gold.
- Amalgamation: Known in Roman times and widely used from the Middle Ages onward (becoming a large-scale process around the 10th century AD), this method used mercury to recover fine gold particles from crushed ore. The gold and mercury formed an amalgam, from which the mercury was later boiled away, leaving the gold behind. This process was hazardous due to toxic mercury vapor.
- Aqua Regia (Chemical Dissolution): This method, using a potent mixture of hydrochloric and nitric acids, was used by alchemists in the Middle Ages. Gold is resistant to most single acids, but aqua regia can dissolve it, leaving some impurities behind. Gold could then be precipitated from the solution as a powder and melted into a bar.
Modern Refining Methods (Late 1800s CE – Present)
The late 19th century brought significant technological advancements that made it possible to refine gold to much higher purities and process low-grade ores efficiently.
- Miller Process (1867): Developed by E.B. Miller, this method transformed refining by introducing chlorine gas into molten gold. Impurities (including silver) turn into chlorides, which float to the surface as slag or vaporize, and are easily removed. This process yields gold of about 99.95% purity and is still widely used today.
- Wohlwill Process (1878): Introduced by Emil Wohlwill, this electrolytic method achieves an even higher purity of up to 99.99% or 99.999%. Impure gold anodes are placed in an electrolyte solution of gold chloride. When an electric current is applied, pure gold is deposited on a cathode, while impurities collect in the solution or as a slime.
- Cyanidation Process (1887): Developed by John Stewart MacArthur, this is primarily an extraction process rather than a refining one, but it is fundamental to modern gold production. It uses a dilute cyanide solution to leach gold from crushed low-grade ores, making large-scale operations possible.
Today, a combination of these processes, along with other advanced chemical and analytical techniques, ensures high efficiency and purity in gold refining.




